Achieving User-Centered Design in Medical Devices
More medical device manufacturers are accounting for user-centered design early in the product engineering process.

BlackHägen Design, a multidisciplinary product design firm, expanded its staff expertise to improve its ability to offer user-centered design for medical devices. Image courtesy of BlackHägen Design.
Latest News
January 26, 2023
Prescription drugs commonly used by seniors today often come with a reversible cap. If the logo is visible on the lid, the cap requires a child-proof squeeze-and-turn to open. If the logo is hidden, it means the lid will open simply by grasping and unscrewing. Seems like a simple thing, but in the world of medical device design, nothing is simple.
Realizing the necessity for senior-friendly caps requires designers to consider the user’s needs with a priority on par with other factors: manufacturability, sourcing, patents, product protection and materials. It could be argued such a cap is intuitive, yet time and again medical device users prove that “intuitive” is never universal.
The medical device industry is undergoing a profound shift based on delivery of care. The traditional approach was primarily clinic based; now the emphasis is on intervention and care in the home or other nonclinical settings. Transparency Market Research claims patient-administered healthcare will grow at an annual rate of 12.5% through 2030. Patient-operated and wearable devices—including devices for activities including vital signs monitoring, sample collection and regimented drug delivery—will only continue to multiply.
These new healthcare delivery settings require medical devices that work equally well in a clinic, an emergency room or a bedroom. The pressure is on for designers to assure successful user interactions in all uses. In practice, this is known as user-centered design (UCD), and it is a rapidly growing engineering discipline.
“We have seen a shift in the medical device design space to include and empower end users to share their insights and expertise in order to inform decisions about design,” says Jeff Morang, lead human factors engineer at BlackHӓgen Design, a Florida-based medical device engineering firm. “This is a pattern that is difficult to ignore.”
“Nearly every time end users have been included early in the design, results in perspectives are changed for everyone involved,” says Bartosz Korec, lead industrial designer at BlackHӓgen. “Taking a UCD approach comes down to realizing that we know what we know and by applying that expertise when creating concepts for end users’ critique and feedback we learn what we don’t know.”
Catching up With the User
Regulatory authorities worldwide are emphasizing usability evaluations for controlling the risks of use-related hazards. The typical waterfall approaches to medical device design are not efficient enough to pass this new standard.
“We have seen time and time again that ‘slowing down’ to get end users’ answers and insights early, often accelerates development. A UCD approach better controls the risks of missing the target of the design, which is to give end users what they really need and want,” Korec says.
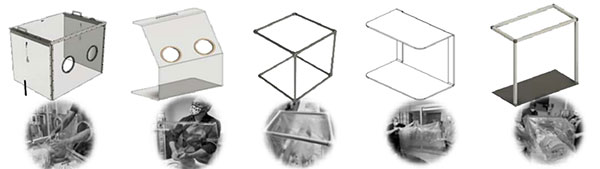
UCD advocates say an increase in collaboration is important, not only among teams in the organization but with regulators as well. BlackHӓgen, for example, endorses regulator review of UCD-derived test data throughout the design process, to evaluate design safety and efficacy, and to reduce or eliminate engineering dead-ends during the final stages of regulatory approval.
One specific act of guidance that emerges from a review of research literature is the need to move beyond the typical design parameters of safety and efficacy and to design for usability. One of the classic texts on product design, Don Norman’s “The Psychology of Everyday Things,” speaks directly to the issue of moving beyond safety and efficacy and into usability. In Norman’s approach, he boils product design down to four factors:
- Make things visible: The need for user interaction must be obvious.
- Provide good mapping: Mapping is the relationship between an action and the intended response.
- Create appropriate constraints: A user-centered device will guide the user in the right direction and, at the same time, limit or eliminate improper actions.
- Design for error: If there is a way to use the device wrongly—or dangerously—a user will find it.
End Users as Design Collaborators
Although the research and industry commentary generally are in agreement that UCD should be integrated early and throughout the entire development process, it seems that few standard methods have emerged from these conversations.
There is the need for collaborative processes, with test patients being part of the collaborative group. A multi-facility study on the creation of a pocket device for tracking health data notes, “involving users has been found to substantially reduce development time because usability problems are identified and resolved before the systems are launched. While UCD is highly acclaimed as a means of ensuring user acceptability, its application with patient users has not been widely disseminated to the health sciences disciplines despite calls for its application.”
An early research project on UCD by J.D. Gould et al. introduced three principles for UCD: Focus on users and tasks early and throughout the design process; measure usability empirically; and design and test usability iteratively. All three principles require direct collaborative involvement by potential end users.
Without a collaborative process, teams often stay siloed and sharing of information is limited, notes BlackHӓgen’s Morang.
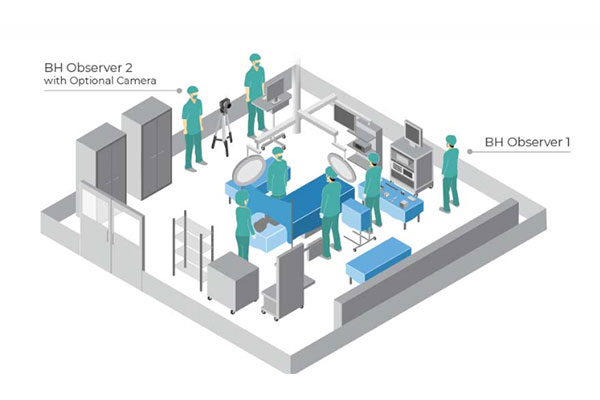
“At best [the lack of collaboration creates] unnecessary delays in design and development due to interruptions and iterations that could have been avoided had end-user insights and feedback been researched and incorporated early,” Morang says. Creating a foundation that identifies early on how users interact with proposed technology or devices “gives proven guidance for the development of a successful and competitive product.”
The methods used by software developers creating smartphone apps can be applied to medical device engineering, says Doug Currie, director of systems engineering at Sunrise Labs, a medical device engineering specialist.
“In the age of patient-centered healthcare, medical device manufacturers must likewise commit to developing a positive user experience and greater ease of operation,” Currie says. “Doing so not only supports patients to make the most of their medical devices, but also generates higher adherence rates and better treatment outcomes.”
Sunrise Labs has developed a set of best practices for user-friendly design of medical devices. The keys are “actively listen to patient comments and recommendations;” to characterize the intended user carefully and fully; and to make every effort to empathize, to “put yourself in the patent’s position.”
Simulation in UCD
The recent pandemic brought home to many engineers the importance of simulation within a user-centric approach to device development. Medical researchers in Atlanta worked with the Georgia Institute of Technology to improve pediatric intubation procedures. Though this is not an example of the patient as the end user, the methods and evaluations are the same.
The team used a dual-iterative process of comparing simulation results with clinical observational data, to create an improved intubation aerosol containment system for use on children. Five separate elements of the system were improved in a series of prototype design/test iterations.
The researchers claim their work was the first study to describe the integration of simulation with human factor ergonomics (HFE) in device development. The study notes, “simulation-based UCD is a patient safety improvement tool uniquely poised at the interface of HFE and UCD. Inefficiencies in the design are identified, and solutions are made to meet end-user needs and minimize risk to patients related to design flaws.”
“Simulation established a common ground that leveled perceptions regarding user needs across disciplines,” the study continues. One big change was seeing how the assumptions of respiratory therapists differed from the actual use of each tested device by physicians. The user experience then informed the next design iteration.
BlackHӓgen’s Morang notes his company uses engineering software to prototype study stimuli for user evaluations, seeking to reflect real-world use. “These can range from hand-built physical models with limited functionality to fully featured alpha designs, which usually mimic production-equivalent designs,” Morang says.
BlackHӓgen has started using virtual reality to evaluate initial models of a new product. “It is very quick to deploy and adaptable for easy modifications,” Korec says. “The high value we see is to use such early prototyping to conduct human factors expert reviews early and prior to conducting simulated use evaluations, which usually are more expensive in terms of time and effort to prepare and execute.”
Subscribe to our FREE magazine, FREE email newsletters or both!
Latest News
About the Author
Randall S. Newton is principal analyst at Consilia Vektor, covering engineering technology. He has been part of the computer graphics industry in a variety of roles since 1985.
Follow DE