Ultimaker Gains Certification as Part of Materialise 510(k) Cleared Mimics inPrint Software
Certified partnership with Materialise delivers safe, reliable 3D printing to point-of-care diagnostics.
November 28, 2018
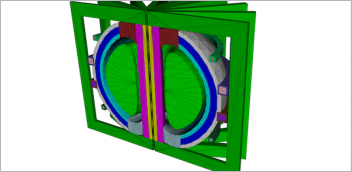
Ultimaker announces that the Ultimaker S5 has been certified by Materialise as a 3D printing solution to create orthopedic, maxillofacial and cardiovascular models for clinical use when used in combination with Mimics inPrint software. The company partnered with Materialise to identify compatibility of the Ultimaker S5 with Materialise Mimics inPrintsoftware to meet FDA regulatory guidelines for point-of-care 3D printing in hospitals.
In 2017, the FDA announced that software intended to create output files for 3D printing patient-specific anatomical models, which are used for diagnostic purposes, is considered a class II medical device and requires regulatory clearance. Materialise was the first company to provide a software that conforms to these regulations and can be used in U.S. hospitals in combination with a compatible 3D printer. After rigorous performance testing and analysis, Materialise and Ultimaker identified that the Ultimaker S5 printer, used in conjunction with PLA and PVA materials, can be safely used with the Materialise Mimics inPrint software for 3D printing models that are used for diagnostic purposes.
“More and more frequently, doctors look to 3D printing for pre-operative planning and the fabrication of physical models for patient management, treatment and surgeon-to-surgeon communications. As diagnostic usage of 3D printers continues to revolutionize patient care, safety and quality remain a top concern for hospitals,” says John Kawola, president of Ultimaker North America. “The Materialise certification of the 3D printing workflow when used with Mimics inPrint reduces the safety- and quality-control burden on doctors and hospitals with it’s clearance by the FDA.”
The medical 3D printing solution leverages software from Materialise, a Leuven, Belgium-based leader in 3D printing software and solutions. When used with Materialise certified materials and specific printers from companies like Ultimaker, Mimics inPrint is the only 3D printing software cleared by the FDA to create anatomical models for patient care. “Starting with a validated system of hardware, software and materials for medical 3D printing helps reduce the burden on hospitals’ quality control programs when introducing the technology,” says Bryan Crutchfield, vice president and general manager of Materialise North America. “This solution we’ve developed with Ultimaker will improve access to 3D printed anatomical models to aid in surgical planning and multidisciplinary team communication.”
Sources: Press materials received from the company.
More Info
Subscribe to our FREE magazine, FREE email newsletters or both!
About the Author
DE’s editors contribute news and new product announcements to Digital Engineering.
Press releases may be sent to them via [email protected].