BMF Gets FDA OK for Ultra Thin Dental Veneer Material
April 23, 2024
Zirconia materials now qualified for use in the production of thin cosmetic veneers, company reports.

3D Systems Gets FDA Clearance for 3D-Printed Cranial Implants
April 15, 2024
FDA clearance enables adoption of 3D Systems' printing system, the EXT 220 MED, with medical-grade PEEK materials to deliver patient-specific cranial reconstruction solutions.
Latest News

CRP USA Elevates Wheelchair Racing Gear for Paralympics Champion
Using Windform XT 2.0 material and selective laser sintering, CRP USA created racing agloves that are lighter, more durable, and offer...

Altair Names Winner of the 2023-2024 Altair Global Student Contest
Student winner used Altair Inspire to slash his team's Shell Eco-marathon vehicle suspension weight by 41%, according to Altair.
Hexagon Unveils Digital Solution to Optimize Factories
By connecting asset digital twins to an accurate and up-to-date Digital Factory, companies can plan and operate more productive, flexible...
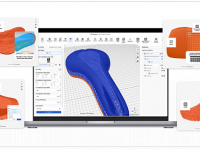
Hyperganic Launches HyDesign
Software reportedly made to democratize 3D-printed lattice design.
Eagle Point Software Partners with SOLIDX to Expand Reach in UK
Collaboration brings Pinnacle Series to SOLIDWORKS users in the UK.
Ohio R&D Center to Advance National Security Propulsion Technology
Purpose of center is to advance additive manufacturing and materials development technology for liquid rocket engines and solid rocket motors.
All posts
New & Noteworthy

New & Noteworthy: Fast, Flexible and Scalable Simulation – In the Cloud
Ansys Access on Microsoft Azure enables seamless deployment of industry-leading simulation tools...

New & Noteworthy: Safe, Cost-Effective Metal 3D Printing - Anywhere
Desktop Metal’s Studio System offers turnkey metal printing for prototypes and...

New & Noteworthy: Direct Neutronics Analysis on CAD
Coreform Cubit 2023.11 workflows enable neutronics directly on CAD for next-generation nuclear energy...

New & Noteworthy: Agile Engineering Collaboration
Authentise Threads is a new software tool for distributed communications and project...
