Simpleware ScanIP Medical Receives FDA 510(k) OK for 3D Medical Printing
Company can 3D print anatomical models with Simpleware ScanIP Medical.
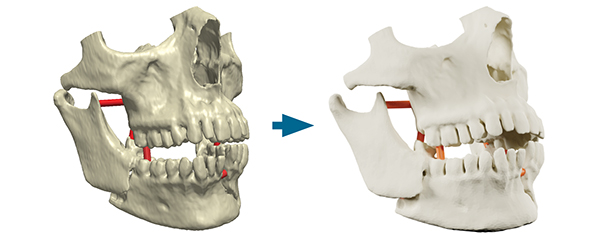
Simpleware ScanIP Medical is intended for use as a software interface and image segmentation system for the transfer of medical imaging information to an output file. Output files can be used for the fabrication of physical replicas using traditional or additive manufacturing method. Image courtesy of Synopsys.
June 23, 2021
Synopsys’ Simpleware ScanIP Medical is now available for 3D medical printing after receiving FDA 510(k) clearance in this area. With the clearance, the software can be used as part of end-to-end DICOM to 3D printing workflows for diagnostic decision-making.
Some of the benefits of the Simpleware solution for Point-of-Care (POC) 3D Printing are as follows:
- Intuitive interface with quick-and-easy access to all tools and features
- Quality anatomical models to practice and plan complex surgical procedures
- Automation of repeatable tasks and operations with scripting and plug-ins
- Direct link to bundled 3D printers guaranteed to work together
- All licenses offering full support from the company's application engineers
- Software cleared for end-to-end diagnostic 3D printing
One of reportedly only a few software programs to have this FDA 510(k) clearance, Simpleware ScanIP Medical is also CE and ISO 13485:2016-certified as a medical device for working with medical imaging data. The FDA 510(k) indications for use are:
Simpleware ScanIP Medical is intended for use as a software interface and image segmentation system for the transfer of medical imaging information to an output file. It is also intended as pre-operative software for diagnostic and surgical planning.
For these purposes, output files can also be used for the fabrication of physical replicas using traditional or additive manufacturing methods. The physical replicas can be used for diagnostic purposes in the field of orthopedic, maxillofacial and cardiovascular applications.
The software is intended to be used in conjunction with other diagnostic tools and expert clinical judgment.
As part of the clearance, Synopsys have partnered with 3D printer companies for a workflow, with more details available here.
“Our technology has always been cutting-edge and best-in-class for working with 3D images and generating 3D models, and it is great to now have regulatory clearance that includes point-of-care 3D printing,” says Kerim Genc, Ph.D., business development manager at Synopsys. “We are excited to deploy these solutions within a point-of-care clinical space, and to leverage our deep technical expertise to provide much-needed solutions to existing bottlenecks in this area.”
Those interested in learning more about the solution can contact the Simpleware team at [email protected] and trial it here.
Sources: Press materials received from the company and additional information gleaned from the company’s website.
More Synopsys Coverage
Subscribe to our FREE magazine, FREE email newsletters or both!
About the Author
DE’s editors contribute news and new product announcements to Digital Engineering.
Press releases may be sent to them via [email protected].
Related Topics