An example of OPMs PEEK material at work. Courtesy of OPM.”
Latest News
August 25, 2014
Although the aerospace and automotive industries have begun to accept additive manufacturing (AM) as a viable and powerful new form of manufacturing, the medical industry has truly embraced the technology. Ever since hearing aid companies saw the value in using AM systems to provide a better product for their customers, the medical field has kept a close eye on 3D printing developments.
Implants and prosthetics are a couple specific areas that have seen extraordinary growth in the last five years. Traditional manufacturing methods have almost no chance of matching the low cost of printing prosthetics, and no other technology is so obvious a fit for bespoke implants. Now, Oxford Performance Materials (OPM) has added facial reconstruction to the list of medical devices built using AM.
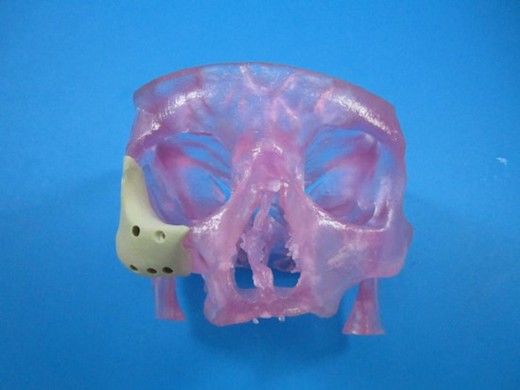 An example of OPM"s OXPEKK material at work. Courtesy of OPM.
An example of OPM"s OXPEKK material at work. Courtesy of OPM.OPM recently received tacit FDA approval to distribute its OsteoFab Patient Specific Facial Device (OPSFD). The OPSFD takes the form of 3D printed sections of OXPEKK (poly-ether-ketone-ketone), which doctors can use in place of missing bone for facial reconstruction surgery. Each piece is laser sintered to match a specific patient using CT imaging data backed up by CAD.
“There has been a substantial unmet need in personalized medicine for truly individualized — yet economical — solutions for facial reconstruction, and the FDA’s clearance of OPM’s latest orthopedic implant marks a new era in the standard of care for facial reconstruction,” said Scott DeFelice, CEO and chairman of OPM. “With the clearance of our 3D printed facial device, we now have the ability to treat these extremely complex cases in a highly effective and economical way, printing patient-specific maxillofacial implants from individualized MRI or CT digital image files from the surgeon.”
In order to get a pass from the FDA, OPM did significant amounts of testing to prove the OXPEKK material is non-toxic and biocompatible. Testing is one particular area where medical AM (and plastic materials) also has an advantage over other industries.
Because of the exacting nature of medical testing, companies are able to draw on extensive background material to prove a product is safe. The same isn’t true for most metal AM, and is something the industry will need to improve moving forward.
Below you’ll find a short video about OPM.
Source: OPM
Subscribe to our FREE magazine, FREE email newsletters or both!
Latest News
About the Author
John NewmanJohn Newman is a Digital Engineering contributor who focuses on 3D printing. Contact him via [email protected] and read his posts on Rapid Ready Technology.
Follow DE